Ian Carmichael

Director - Notre Dame Radiation Laboratory
University of Glasgow, Scotland, B.Sc.Hons (1971) Ph.D. (1974)
Phone: (574) 631-4502
Email: carmichael.1@nd.edu
Office: 321 Radiation Research Building
Theoretical Studies of Reactive Intermediates
Scientific Interests
Quantum Chemistry of Reactive Intermediates
Structure and properties of radicals, radical ions, and excited states in solution and on surfaces.
Magnetic Interactions in Molecular Species
EPR splittings; NMR spin-coupling constants and chemical shifts; nuclear quadrupolar couplings.
Theory of Radiation and Photochemical Transformations
Photophysical processes; dissociative electron attachment; transient absorption spectroscopy.
Radiation Damage in Macromolecular Crystallography
Specific damage mechanisms and mitigation strategies.
Recent Accomplishments
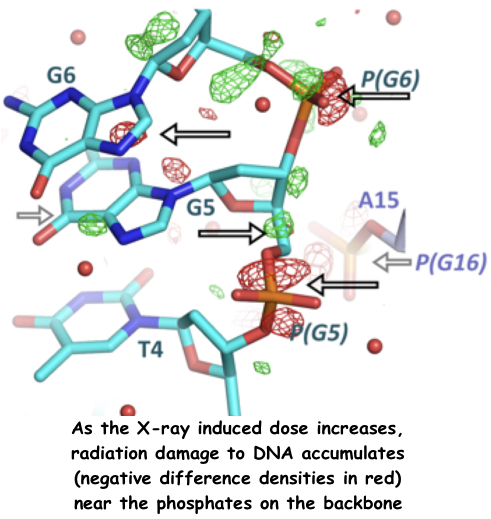
Radiation damage in MX
Systematic investigation of radiation damage to a crystal of a DNA 16-mer diffracting to 1.8 Å and held at 100 K, was probed up to an absorbed dose of 45 MGy. The DNA crystal was found to be fairly radiation insensitive to both global and specific damage.
In terms of specific damage, the regions of DNA found to be particularly susceptible were those associated with certain bound calcium ions, sequestered from the crystallization buffer. Damage was particularly evident in electron density maps around the backbone phosphate groups, appearing on the nucleobases only at the highest doses.
Understanding dissociative electron attachment
Despite decades of gas-phase studies on dissociative electron attachment (DEA) to various molecules, as yet there has been no direct detection and characterization of the neutral radical species produced by this process. We use stepwise electron spectroscopy to directly measure and characterize the neutrals produced upon zero-electron-energy DEA to a model molecule, carbon tetrachloride. We observe the direct yield of the trichloromethyl radical and measure the appearance energies of all the other neutral species. Combined with high-level quantum chemical calculations this allows a comprehensive analysis of both the initial DEA event and the subsequent electron-impact ionizations under single-collision conditions. This work paves the way toward a complete experimental characterization of DEA processes, in turn providing a refined understanding of the low-energy electron-induced formation of radical species.
NMR spin couplings in biomolecules
NMR J-couplings measured by selective 13-C labeling of twelve, structurally-diverse,β(1→4) linked disaccharides were analyzed using parametrized equations obtained from density functional theory calculations, coupled with Fredholm theory and circular statistics. Our statistical program, MA’AT, allowed the modeling of the linkage torsional parameters, phi (φ) and psi (ψ),as a single von Mises distribution with mean positions in close accord to those of rotamer populations obtained from 1 μs aqueous molecular dynamics (MD) simulations and crystallographic database statistical analyses. An experimental means to validate the conformational preferences predicted from MD simulations is thus established.
Selected Publications
Chakraborty, D.; Kharchilava, G.; Carmichael, I.; Ptasińska, S. “Dissociative electron attachment studies of gas-phase acetic acid using a velocity map imaging technique”. J. Phys. B-At. Mol. Opt. Phys. 56 (2024) 245202 link
Lisouskaya, A., O. Schiemann, and I. Carmichael. "Unveiling the mechanism of photodamage to sphingolipid molecules via laser flash photolysis and EPR" Photochem. Photobiol.99 (2023) 1400-11 link
Lisouskaya A., U. Markad, D.M. Bartels and I. Carmichael. "Reactivity of Zn+aq in high-temperature water radiolysis" Phys. Chem. Chem. Phys. 24 (2022) 19882 link
Lisovskaya,A., I. Carmichael, and A. Harriman. "Pulse radiolysis investigation of radicals derived from water-soluble cyanine dyes: Implications for super-resolution microscopy" J. Phys. Chem. A 125 (2021) 5779-93 link
Lisovskaya, A., O. Shadyro, O. Schiemann, and I. Carmichael. "OH radical reactions with the hydrophilic component of sphingolipids" Phys. Chem. Chem. Phys. 23 (2021) 1639-48. link
de la Mora, E., N. Coquelle, C.S. Bury, M. Rosenthal, J.M. Holton, I. Carmichael, E.F. Garman, M. Burghammer, J.-P. Colletier, and M. Weik. “Radiation damage and dose limits in serial synchrotron crystallography at cryo- and room temperatures” Proc. Natl. Acad. Sci. USA 11 (2020) 4142-51 link
NMR-related (recent)
Zhang W.H.; R.J. Meredith; X.C. Wang; R.J. Woods; I. Carmichael; A.S. Serianni "Does inter-residue hydrogen bonding in β-(1→4)-linked disaccharides influence linkage conformation in aqueous solution?" J. Phys. Chem. B 128 (2024) 2317-25. link
Meredith R.J.; M.K. Yoon; I. Carmichael; A.S. Serianni " MA'AT analysis: Unbiased multi-state conformational modeling of exocyclic hydroxymethyl group conformation in methyl aldohexopyranosides" J. Phys. Chem. B 128, (2024), 2360–70. link
Meredith, R.J., I. Carmichael, R.J. Woods,and A.S. Serianni "MA'AT analysis: Probability distributions of molecular torsion angles in solution from NMR spectroscopy" Acc. Chem. Res. 56 (2023) 2313–28. link
Tetrault, T., R.J. Meredith, M.K. Yoon, C. Canizares, A.G. Oliver, I. Carmichael, and A.S. Serianni. One-bond 13C-13C spin-coupling constants in saccharides: A comparison of experimental and calculated values by density functional theory using solid-state 13C-NMR and X-ray crystallography" Phys. Chem. Chem. Phys. 25 (2023) 16048-59. link