Prashant V. Kamat

Karnatak University, B.S. (1972)
Bombay University, India, M.S. (1974) Ph.D (1979)
Phone: (574) 631-5411
Email: Kamat.1@nd.edu
Office: 223B Radiation Research Building
Charge Transfer Processes and Energy Conversion
Scientific Interests
Light Harvesting Assemblies
Elucidation of excited state dynamics, energy and electron transfer, and surface chemistry of semiconductor quantum dots and metal halide perovskite nanocrystal based assemblies.
Photocatalysis
Interfacial charge transfer at semiconductor and metal interface, role of metal nonoparticles as co-catalysts in photocatalysis and design of hybrid assemblies for light energy conversion.
Electrochemistry at the Mesoscale
Evaluation of electrocatalytic and photocatalytic processes and modulation of charge transfer at the electrode/electrolyte interface through applied bias.
Recent Accomplishments
Energy Transfer in Semiconductor-Molecular Hybrids
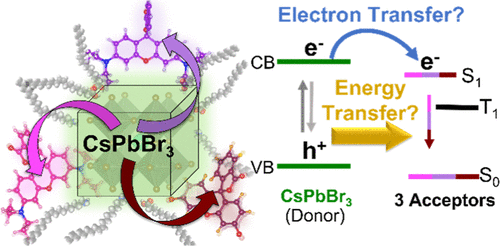
Energy and electron transfer interactions are dictated by aspects of both the nanocrystal donor and the chromophoric acceptor molecules. For the donor, nanocrystal composition can modulate spectral overlap, the surface ligand environment can influence acceptor binding and electron transfer efficiency, and the nanocrystal size can allow for greater wavefunction overlap. Each of these properties influences the excited state pathway in both energy and electron transfer processes. As seen in this study, the properties of the acceptor play a crucial role as well. Stronger binding of functionalized acceptors allows for efficient excited-state interactions at low concentrations of the added acceptor. Depending on the propensity of aggregation for acceptors, the nanocrystal surface may allow for the assembly of acceptors without aggregation. Despite both singlet and triplet energy transfer being thermodynamically favorable, only singlet energy transfer is found to occur between the donor CsPbBr3 and the rhodamine-based acceptor molecules. However, the pendant groups decorating the acceptors are found to directly influence the excited state process by modulating the chromophore’s binding, degree of aggregation, and the observed rate constant of energy transfer (kEnT). Additionally, we find that a subpopulation of each acceptor studied engaged in electron transfer as a competitive process to singlet energy transfer. The study provides important insights for tuning the interactions between nanocrystals and organic chromophores for light harvesting, and highlight the complexity of excited state energy and charge transfer processes.
Photoinduced Electron Transfer across CsPbBr3 Nanocrystal Interface
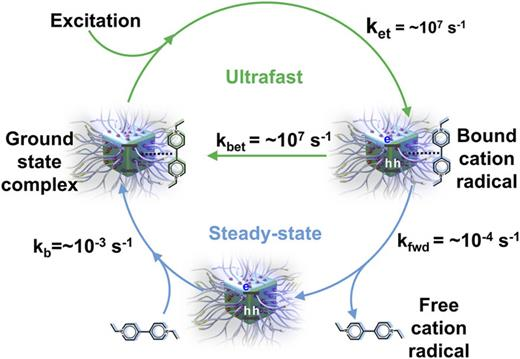
Polymethylmethacrylate-capped CsPbBr3 nanocrystals exhibit greater tolerance to the increased polarity of the medium (toluene:ethanol). The viologen molecules bind strongly to PMA-capped CsPbBr3 nanocrystals and facilitate excited state electron transfer. The strong binding of viologen to CsPbBr3–PMA nanocrystals allows the initial charge separation efficiency as high as 70%. The charge-separated pair has a lifetime of microseconds before undergoing charge recombination. A significant fraction of the charge-separated pair escapes the initial charge recombination and produces a net yield of electron transfer product (viologen radical) under steady-state irradiation. Although the initial electron transfer from the excited semiconductor into the electron acceptor such as viologen moiety is an ultrafast process, it is the back electron transfer process that occurs at different time scales that eventually determines the net electron transfer yield. Hence, in photocatalytic studies, it is not sufficient to demonstrate just the forward electron transfer. It is equally important to estimate various back electron transfer steps that ultimately determine the effectiveness of a photocatalyst for photoinduced electron transfers.
Vectorial Charge Transfer across Photocatalytic Membrane
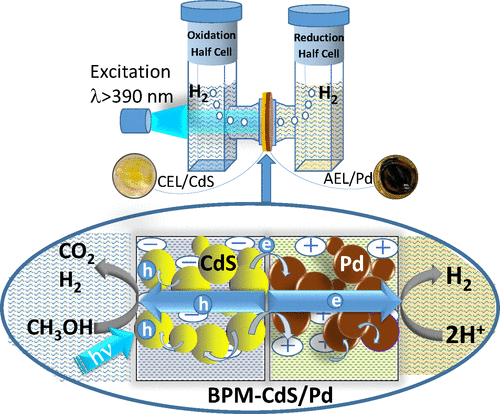
A bipolar membrane with specific cation exchange and anion exchange properties between the two layers enables design of a photocatalytically active membrane that also separates reaction compartments. The key advantage of such a photoactive membrane lies in the separation of oxidation and reduction processes while continuing to engage in the photodriven reactions. Such a concerted design of photocatalytic membrane allows “vectorial” electron and hole transfer between semiconductor-metal layers. The example of simultaneous oxidation of 4-chlorophenol and the reduction of 4-nitrophenol under visible light irradiation of the BPM-CdS/Pd shows its effectiveness in photocatalytic remediation of chemical contaminants. This approach of separating oxidation and reduction compartments using a bipolar photocatalytic membrane has also been utilized to assess the contribution of sacrificial donors in photocatalytic H2 production. The oxidation of sacrificial donors (Na2S, ascorbic acid, or TEOA) contributes >30%to the net H2 generation. Na2SO3 was the only sacrificial donor which did not directly contribute to the H2 generation. Thus, the choice of sacrificial donor and its oxidation in photocatalysis is important in determining the photocatalytic efficiency, as it can significantly alter the measurements of H2 production in semiconductor-assisted photocatalysis.
Selected Publications
DuBose, J.T., P.V. Kamat. "How Pendant Groups Dictate Energy and Electron Transfer in Perovskite–Rhodamine Light Harvesting Assemblies." J. Am. Chem. Soc. 145 (2023) 4601–4612.
DuBose, J.T., A. Christy, J. Chakkamalayath, P. V. Kamat. "Trap or Triplet? Excited State Interactions in 2D Perovskite Colloids with Chromophoric Cations." ACS Nano. 16 (2023).
Chakkamalayath, J., N. Hiott, P.V. Kamat. "How Stable Is the 2D/3D Interface of Metal Halide Perovskite under Light and Heat?" ACS Energy Lett. 8 (2023) 169–171.
Kipkorir, A., X Jin, H. Gao, P.V. Kamat. "Photoinduced electron transfer across the polymer-capped CsPbBr3 Interface in a Polar Medium." J. Chem. Phys. 158 (2023) 144702.
DuBose, J.T., P.V. Kamat. "Energy Versus Electron Transfer: Managing Excited-State Interactions in Perovskite Nanocrystal–Molecular Hybrids." Chem. Rev. 122 (2022) 12475–12494.
DuBose, J.T., P.V. Kamat. "Hole Trapping in Halide Perovskites Induces Phase Segregation." Acc. Mater. Res. 3 (2022), 761–771.