A simple structural motif has been computationally uncovered that well reproduces all the observed experimental properties of the excess electron in water.
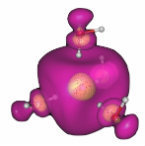
The structure of the hydrated electron - an excess electron in water - has been the subject of wonderment and fierce debate since its first observation in the 1960s. In collaboration with researchers at Oakland University, NDRL researchers Jonathan Walker and David Bartels recently found a very simple molecular motif for the hydrated electron having properties in close accord with all of the experimental structural information. Their minimal anion cluster model comprises four water molecules, tetrahedrally positioned around a central void, each one with one OH bond oriented toward the center. The unpaired electron fills the central void, strongly overlaps the four surrounding waters, and extends into the second solvation shell. This work has just been published in the Journal of Physical Chemistry A and is titled, "A Simple Ab Initio Model for the Hydrated Electron That Matches Experiment."
Shown in the Figure: Computed spin density - outer surface (violet) encloses 80% and the inner surface (yellow) 20% - of the excess electron in water.