Aliaksandra Lisouskaya

Aliaksandra Lisouskaya
Belarusian State University, Minsk, Belarus, M.Sc. Chem.(2009), Ph.D.(2012)
Phone: (574) 631-5457
Email: alisousk@nd.edu
Office: 105C Radiation Research Building
Radiation Chemistry and Photochemistry in Aqueous Solutions
Scientific Interests
Pulse Radiolysis of Aqueous Media
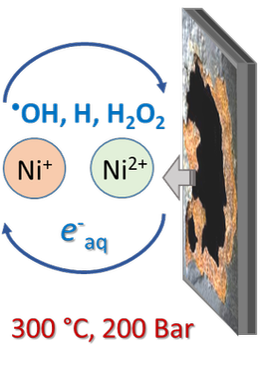
Kinetics and mechanisms of radical reactions of nuclear reactor water under high temperatures and pressures. Pulse radiolysis of transition metal ions over wide temperature ranges to solve radiolytic corrosion problems for both current and future generation reactors.
Radiation Chemical Impacts on the Nuclear Waste Stream
Radiolytic stability of solvents and organic ligands used in separation systems in nuclear waste reprocessing. Time resolved EPR spectroscopic techniques to establish identity and kinetics of transients from organic ligands used in solvent extraction processes.
Free Radical and Redox Chemistry of Bioorganic Compounds
Mechanisms of free radical reactions of organic compounds and biomolecules probed by pulse radiolysis, flash photolysis and EPR spectroscopy that have applications ranging from those relevant to the nuclear power industry to photochemistry of importance in biology and medicine.
Back to top
Recent Accomplishments
Reactions of nickel ions in water radiolysis up to 300°C
Radicals from tributyl phosphate decomposition: a combined electron paramagnetic resonance spectroscopic and computational chemistry investigation
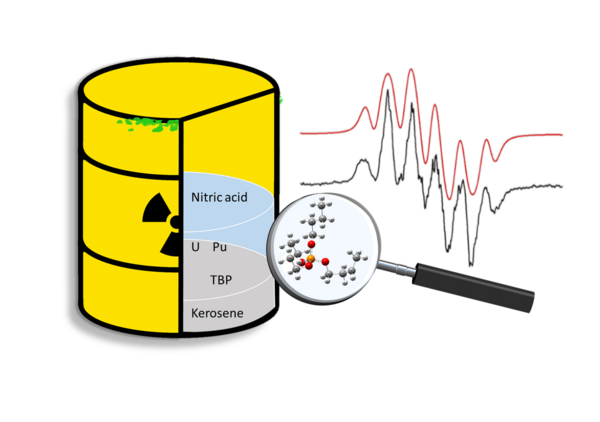
This study presents experimental data obtained by the methods of electron paramagnetic spectroscopy and computational chemistry for studying the structure of radicals derived from tributyl phosphate (TBP) under irradiation. TBP is exposed to high doses of ionizing radiation during nuclear waste separation and it is necessary to understand its radiolytic degradation pathways. We applied spin-trapped EPR to estimate radiation-chemical yields of TBP radicals formed in situ under X-ray irradiation at room temperature. To find the structure of radicals formed from TBP, we use low-temperature EPR. To study the reactions of TBP with the OH radical in aqueous solutions, we used the continuous flow EPR method at room temperature, which also makes it possible to elucidate the radical kinetics. An automatic conformational analysis on TBP-derived radicals was performed to investigate the possible radical conformations. The obtained data make it possible to estimate the radiation resistance and determine the structure of radical conformers induced by irradiation with X-rays or electron beams, and chemically generated.
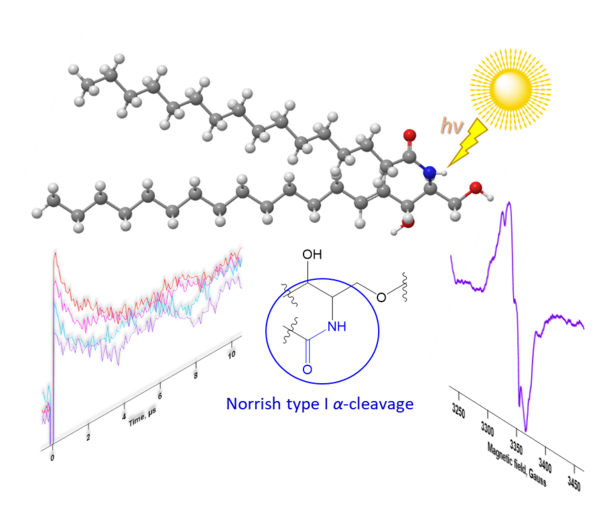
Unveiling the mechanism of photodamage to sphingolipid molecules via laser flash photolysis and EPR
Selected Publications
Sosulin I.S., D.H. Ryan, A. Lisouskaya. "Radicals from Tributyl Phosphate Decomposition: a Combined Electron Paramagnetic Resonance Spectroscopic and Computational Chemistry Investigation" Phys. Chem. Chem. Phys. (2023)
Lisouskaya A., U. Markad, D.M. Bartels. "Reactions of Nickel Ions in Water Radiolysis up to 300 °C." J. Phys. Chem. B, 127 (2023) 2784–91.
Lisovskaya A., O. Schiemann, I. Carmichael. "Unveiling the Mechanism of Photodamage to Sphingolipid Molecules via Laser Flash Photolysis and EPR" Photochemistry and Photobiology (2023)
Lisouskaya A., U. Markad, I. Carmichael, D.M. Bartels "Reactivity of Zn+aq in High-Temperature Water Radiolysis" Phys. Chem. Chem. Phys. 24 (2022) 19882–9.
Lisouskaya, A., P. Tarábek, D.M. Bartels, I. Carmichael "Persistent Radicals in Irradiated Imidazolium Ionic Liquids Probed by EPR Spectroscopy" Rad. Phys. Chem. 202 (2022) 110513.
Lisovskaya A., I. Carmichael, A. Harriman. (2021) "A Pulse Radiolysis Investigation of Radicals Derived from Water-Soluble Cyanine Dyes: Implications for Super-Resolution Microscopy" J. Phys. Chem. A, 125 (2021), 5779–93.