Sylwia Ptasinska

Maria Curie-Sklodowska University, Poland. M.Sc. (2001)
Leopold-Franzens-University, Austria. Ph.D. (2004)
Leopold-Franzens-University, Austria. Habilitation (2011)
Phone: (574) 631-2819
Email: sptasins@nd.edu
Office: 101 Radiation Research Building
Physical and Chemical Processes in Biosystems and on Semiconductor Surfaces
Scientific Interests
Heterogeneous Processes on Surfaces
Photoelectron spectroscopy, electronic and chemical structures, water/semiconductor interfaces
Non-Thermal Plasma Radiation
Plasma diagnostics, plasma reactive species, plasma effects on molecular systems
Low-Energy Electron Interactions
Dissociative electron attachment, electron ionization, mass spectrometry
Recent Accomplishments
Toward a realistic description of oxidized solid/liquid interfaces
We integrated computational-experimental approach for understanding chemistry at complex solid-liquid interfaces. Ambient-pressure X-ray Photoelectric Spectroscopy (XPS) and high-level first-principles methods were employed to understand the specific relationship between surface chemistry and key electronic (e.g., band edge) and spectroscopic (e.g., XPS) features at the H2O and InP(100) or GaP(100) interfaces. Combination of ambient–pressure XPS, band edge measurements, and the theoretical calculations was used to deduce that dissociated and non-dissociated adsorbed water coexist on the GaP and InP surfaces, altering surface dipolar properties. We provided the link between interfacial electronic properties and local chemistry, which is necessary for devising meaningful strategies to improve photoelectrochemical cell’s performance and durability.
Ring opening in five-membered rings induced by gentle impact of low energy electrons
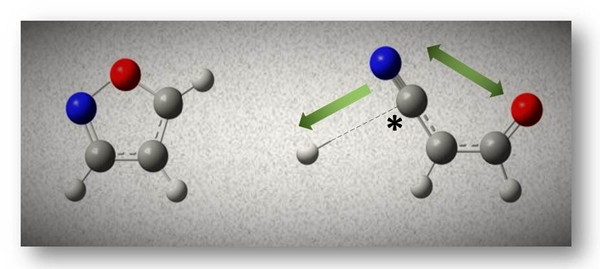
Our experimental and computational approaches revealed that the N-O bond on the five-membered ring structure in isoxazole is highly susceptible to DEA. Substitution of the –CH= group of furan with the N atom in isoxazole enhance electron scavenging ability at low electron energies, whereas in furan dissociative electron attachment (DEA) occurs only via core-excited resonances; Our comparative study of isoxazole, 3-methylisoxazole, and 3,5-dimethylisoxazole revealed the fragmentation pathways that lead to each anion product formed due to DEA. Ring opening patterns can be induced by DEA to isoxazole. The most dominant fragmentation pathway that formed an [isoxazole-H] anion via hydrogen loss at carbon site (indicated by * on the picture) was initiated by the lowest shape resonance state and led to ring opening. With the assistance of quantum chemistry calculations, several reaction pathways also were considered and justified by calculating the enthalpies of reactions; Upon DEA, oxazole, which is an isomer of isoxazole, and thiazole in which the oxygen is replaced by sulfur, also exhibited ring opening. Both compounds showed similar fragmentation patterns through corresponding bonds in both molecules. Five-membered heterocyclic structures, which exist widely in many chemical systems can be easily transformed in various processes involving radiation.
Selected Publications
Zhang, X., B.C. Wood, A.J.E. Rowberg, T.A. Pham, T. Ogitsu, J. Kapaldo, S. Ptasinska. "Kinetically versus thermodynamically controlled factors governing elementary pathways of GaP(111) surface oxidation." J. Power Sources, 560 (2023) 232663. link
Chakraborty,D., D.S. Slaughter, S. Ptasinska. "Dynamics of resonant low-energy electron attachment to ethanol-producing hydroxide anions." Phys. Rev. A 108 (2023) 052806. link
Sebastian, A., D. Lipa, S. Ptasinska. "DNA strand breaks and denaturation as probes of chemical reactivity versus thermal effects of atmospheric pressure plasma jets." ACS Omega 8, (2023) 1663–70. link
Sebastian, A., D. Spulber, A. Lisouskaya, S. Ptasinska. "Revealing low-temperature plasma efficacy through a dose-rate assessment by DNA damage detection combined with machine learning models." Sci Rep 12 (2022) 18353. link
Ptasinska, S., Varella, M.T.d., Khakoo, M.A., Slaughter D, Denifl S. "Electron scattering processes: fundamentals, challenges, advances, and opportunities." Eur. Phys. J. D 76 (2022) 179. link
Chen, B., S. Ptasinska, P.V. Kamat. "Metal cocatalyst dictates electron transfer in Ag-decorated MoS2 nanosheets." J.Phys. Chem. C 126 (2022) 11907-14. link